University of Illinois, Dept of Biochemistry
HOW DOES ESTROGEN INDUCE PROLIFERATION OF CANCER CELLS?
SMALL MOLECULE INHIBITORS OF ESTROGEN AND PROGESTERONE RECEPTOR ACTION IN BREAST CANCER CELLS
SMALL MOLECULE INHIBITORS OF ANDROGEN RECEPTOR ACTION IN PROSTATE CANCER CELLS
REGULATION OF IMMUNE SURVEILLANCE AND APOPTOSIS BY PROTEINASE INHIBITOR 9
mRNA Binding Proteins
mRNA BINDING PROTEINS
Collaborators: Dr. Ho-Hyung Woo and Dr. Setsuko Chambers (Univ of Arizona)
Alumni: Dr. Robin Dodson, Dr. Kathryn Flavin, Dr. Hiroshi Kanamori, Sherise Gipson
Researchers: Lily Mahapatra and Dr. Chengjian Mao
Background
The level of an mRNA in the cytoplasm represents a balance between the rate at which the mRNA precursor is synthesized in the nucleus, and the rates of nuclear RNA processing and export, and cytoplasmic degradation. Long ago, we showed that estrogen induction of the mRNA encoding the egg yolk precursor protein vitellogenin in liver cells of Xenopus laevis increased the half-life of vitellogenin mRNA from 16 hours to 500 hours (about 3 weeks). This work helped establish the regulation of mRNA stability as a major regulatory site in vertebrate cells. We identified the estrogen-inducible mRNA binding protein vigilin as playing a key role in this process. The role of vigilin in regulation of vitellogenin mRNA stability is shown in schematic form in the figure.
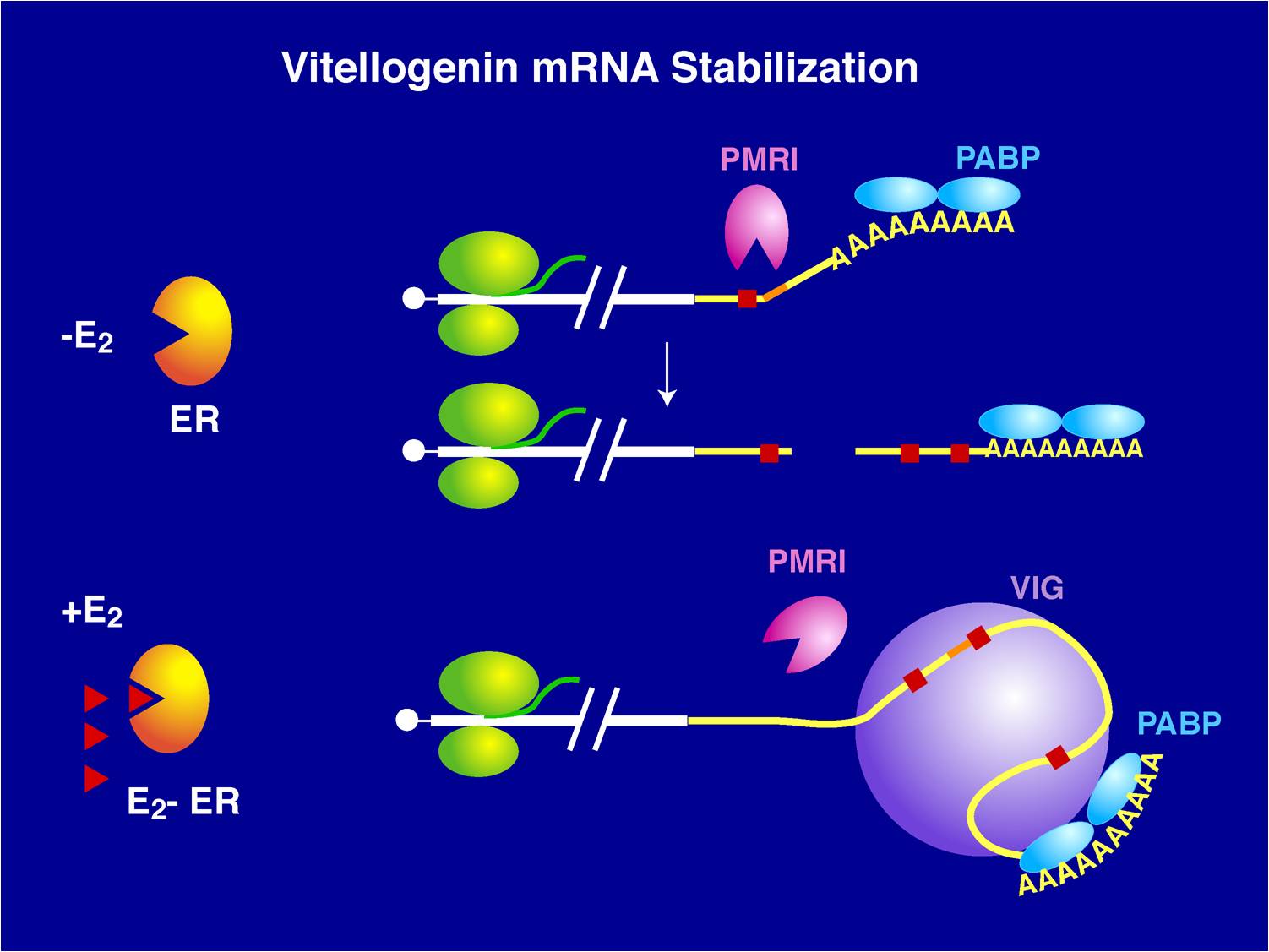
Vigilin in mRNA metabolism
Vigilin is highly conserved in all organisms from yeast to humans. Among RNA binding proteins vigilin is unique in that it contains 15 K-homology (KH) RNA binding domains (Goolsby KG, et al., (2003) Nucleic Acids Res., 31:5644-5653). Although each individual vigilin domain binds weakly to a short 4-5 nucleotide recognition sequence, by summing up the large number of weak interactions of its many KH domains vigilin binds with high affinity to its vitellogenin mRNA recognition sequence (Mao C, et al., (2006) Anal Biochem, 350:222-232). Importantly, vigilin binds to the mRNA encoding the protoconcogene c-fms in breast cancer cells and reduces c-fms mRNA stability by competing for the binding site with another RNA binding protein called HuR which stabilizes c-fms mRNA and thereby destabilizes c-fms mRNA (Woo et al., (2011) Mol. Cell Biol, 31:215-225).
Our data suggests that vigilin may play a role as a hunger signal and that in normal circumstances its level is relatively fixed in human cells. We are using the skills gained in our work with vigilin to study a different mRNA binding protein that is overexpressed and is associated with reduced survival in several types of human cancer.
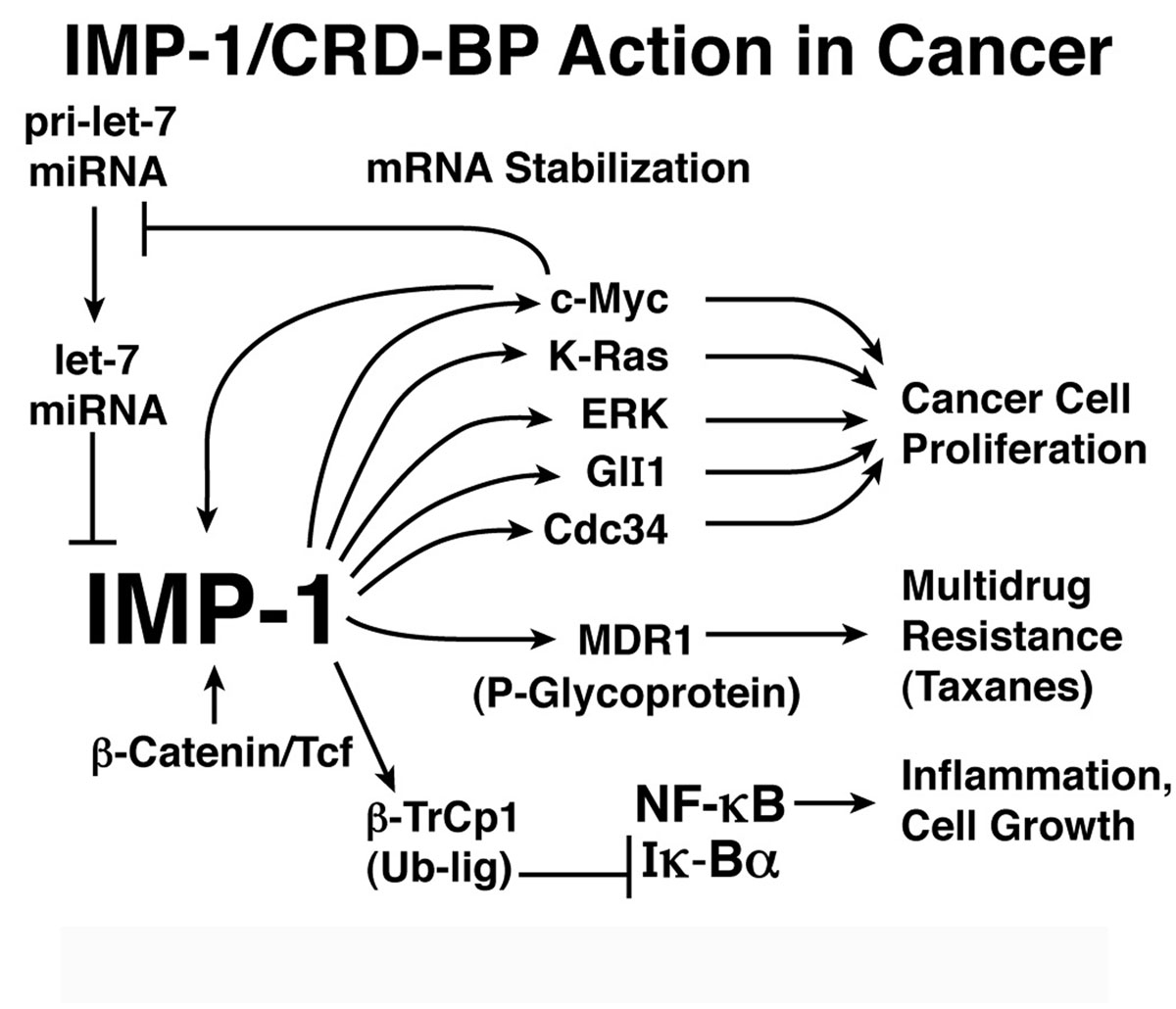
IMP-1 is an RNA binding protein implicated in cancer
IMP-1/CRD-BP/IGF2BP1 and IMP-3 are mRNA binding proteins that bind to and stabilizes the mRNAs encoding c-myc, K-Ras, ERK and other oncogenes, the tumor enabling factor NF-kB and multidrug resistance factor 1 (MDR1), enolin, which stimulates cancer cell migration and invasion and other proteins important in the growth and metastases of cancer. Elevated expression of IMP-1 and IMP-3 is associated with increased growth of cancer cells, resistance to anticancer drugs and a poor prognosis in most cancers (lung, colon, ovarian, pancreatic, renal and others). Consistent with an important role in cancer IMP-1 is up-regulated by c-Myc and b-catenin and it is a major regulatory target of let-7 microRNA. Reduced expression of let-7 microRNA may be the second most common change in human cancer. Although it is overexpressed in many cancers, little is known about regulation and function of IMP-3.
We are carrying out studies of the interaction of IMPs with their mRNA regulatory targets. We developed a high throughput screen for small molecule inhibitors of IMP-1. These inhibitors will be important probes for studying the actions of IMP-1 at key cancer-related targets and will be potential candidates for further therapeutic development.
Selected Publications
Goolsby KM and Shapiro DJ (2003) RNAi-mediated depletion of the 15 KH domain protein, vigilin, induces death of dividing and non-dividing human cells but does not initially inhibit protein synthesis. Nucleic Acids Res, 31:5644-5653.
Mao C, Flavin KG, Wang S, Dodson R, Ross J, and Shapiro DJ (2006) Analysis of RNA-protein interactions by a microplate-based fluorescence anisotropy assay. Anal Biochem, 350:222-232.
Woo H-H, Yi X, Lamb T, Menzl L, Baker T, Shapiro DJ, Chambers SK (2011) Posttransriptional suppression of protoconcogene c-fms by vigilin in breast cancer. Mol. Cell Biol, 31:215-225. PMCID: 3019847.
Mahapatra L, Mao C, Andruska N, Zhang C, Shapiro DJ. (2014) High-throughput fluorescence anisotropy screen for inhibitors of the oncogenic mRNA binding protein, IMP-1. J. Biomolecular Screening 19(3): 427-36.